Pax Herbal Clinic and Research Laboratories, Ewu, Edo State, Nigeria, has reported that NAFDAC has approved their Herbal remedy SAFE FOR PUBLIC USE.
In an internal memo that went viral on social media, it was said that, “after a series of screening, spanning eight weeks, NAFDAC has today approved our PAXHERBAL Covid-19 herbal drug, PAXHERBAL CUGZIN, for public use. It is the first to be so approved and presently the only one. The drug is specifically for treating the symptoms associated with the coronavirus.”
Fada Kay got in touch with one of the Benedictine Monks, who verified the story as authentic.
Writing on their official Facebook Page, under the caption OFFICIAL ANNOUNCEMENT FROM PAXHERBAL, Fr Adodo said, “We at Pax herbals are happy to confirm that our CVD PLUS, which has been renamed CUGZIN, has been issued a NAFDAC number, as ‘an immune booster and anti-infective’. We are aware that there is a lot of anxiety in the land and people are hungry for any reliable immune booster as prevention. PAXHERBAL CUGZIN will help to boost body immunity, as there is yet no officially approved drug for the cure of COVID-19. We ask our followers to look out on our Facebook page for official statements and be weary of different claims or stories or images on social media about our products.”

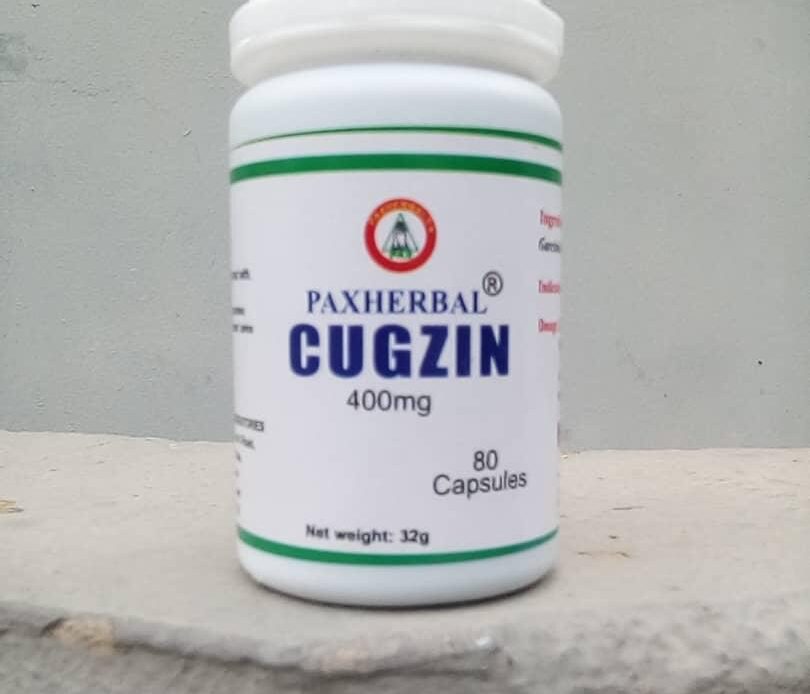
The herbal remedy had earlier been produced under the brand name of “CVD PLUS” before it was rebranded to “PAX HERBAL CUGZIN”. The novel drug is in capsules form, and is packaged in a container of eighty 290mg capsules, each containing Garcinia Kola, Curcuma Longa, and Zingiber officinale.
Pax Herbal went further to state that the Herbal Cugzin can serve as an immunity booster, as well as an anti infective. PAXHERBAL further noted that it was not to be used by pregnant women and children under 11 years old.
Recall that on April 29 2020, the Pax Herbal Clinic and Research Laboratories, released a Press Statement on it’s official Facebook Page. The statement was “ABOUT CURE FOR COVID-19”, though the claim was really about the “TREATMENT” of COVID-19.
In that release, which we ran under the caption “Catholic Monks Discover ‘CVD PLUS’ for the Treatment of COVID-19“, we noted, among other things, that “Fr. Anslem Adodo, Director at Pax Herbals had earlier recommended some of its already NAFDAC-approved drugs for the management of symptoms associated with COVID-19.
In an interview granted to Catholic Herald; official newspaper of the Catholic Archdiocese of Lagos, Rev. Fr. Dr. Anselm Adodo, whose doctoral degrees are in Management of Technology and Innovation, and Medical Sociology said, “we have herbal medicines for many of the diseases that plague our people. For more than 25 years, we have been producing herbal medicines for Diabetes, Hypertension, Asthma, Malaria, prostrate problem, male and female infertility and others. For those 25 years, we have strived to maintain the same high standard and consistency. The only thing we are poor at is making noise about what we do. Those who use the products make the noise for us” (cf. fadakay.org).
Now that the Pax Herbal Cugzin has gained approval as SAFE FOR USE, it is important to warn the general public not to self-medicate. There are Pax herbal distributors trained to answer all questions regarding usage of the Novel drug. Hence, PAXHERBALS will not be held liable for abuse of this drug, should people decide to overdose on it, or use without proper recourse to professional guidance.
All Pax Herbal products are available online HERE.
DISCLAIMER
NAFDAC has issued an official DISCLAIMER. The purpose of this disclaimer is for the general public to note that NAFDAC approval means that the Herbal Remedy has been considered SAFE FOR USE. NAFDAC approval does not automatically mean that the claims of the said drug are automatically verified.
The public is advised to NOTE this difference, as NAFDAC is only a regulatory body. It’s function is not to APPROVE or DISAPPROVE claims.

Good one
I will like to have these drugs how can I get it way billed to me or the nearest shop where I can purchase it in Port Harcourt, Rivers State. I will also want the Pax Herbal bitters as it’s very helpful to my husband. He is diabetic
This is good news…so how can one access this medication…who can one call, which pharmacy stores across the nation can one visit – I mean on a state by state basis…?
Dr Okoro Victor
I am very happy to hear and read that NAFDAC has eventually approved the Herbal medicine to boost and cure CORONAVIRUS. I give Glory to God for this great achievements. May God bless and protect all the Catholic Rev. Fathers involved in this great breakthrough.
May the almighty God be blessed for this breakthrough. I have been searching for your products since I used one approximately ten years ago.
How can I be your distributor at
OYIGBO, RIVERS STATE
I am Dr Okoro Victor
DIVINE WISDOM HOSPITAL LTD
SATELLITE VILLAGE ALONG PH-ENUGU EXPRESS ROAD OYIGBO RIVERS STATE
08033394222
For the covid 19 herbal drug approval by NAFDAC, oh! What a relief to humanity!
We thanks and praises to the Almighty God for prayer answered.
Frid Omosigho-osunde
Thanks for this post. For those who want to know where to buy the new anti-COVID-19 herbal supplement from Paxherbals, I just ordered mine from the website http://www.paxyou.com. It seems the demand is very high so they placed the product on pre-order as at the time of writing
https://www.paxyou.com/product/paxherbal-cugzin-anti-covid-19-supplement/
Chapel of the Healing Cross opposite LUTH
We’re very grateful for all you are doing for Man kind and our country in general. We pray that God will continue to give you people the knowledge and wisdom to discover more hidden remedies for our diseases. Above all, our goes to God Almighty who gave you the strength and knowledge to do all that you are doing . Our amiable Rev. Fr. Dr. Anslem we bless you Fr. Continue to do your good work, God bless all of us in Jesus Name Amen.
Padre, Congratulation to our catholic faithful for another milestone to the church. I am proud to be a catholic. You people should keep it up & keep the flag frying. The PAX HERBAL is a hope for nigerians and the own world.
Great news of the century, thank God that this breakthrough is Handy to save million souls in Nigeria and beyond. Am very delighted that this divine breakthrough is rooted from the Catholic Church. Am very proud to be a Catholic. God bless us, amen.
Hummm! I will rather advise we hear from NAFDAC themselves before placing order and buying. This report says nothing has been heard from them. Let us not be too hasty in our conclusions.
What are the ingredients? Some people might be allergic to some of the combined herbs. Just a thought.
Thank God for this great discovery, please how can we get in warri, is very necessary for us ooooo. Up Catholic Faithful ooooo
Realy glad to be a son of Esanland right now. Please do not jump the gun. Let NAFDAC release a statement first before ordering. I suggest the monastery expand their facilities to enable them cope with the expected demand for the drug. God bless you all at Paxherbal.
Hallelujah. All glory to our God who gave the inspiration. It is written, “He created the herbs for the service of man.”
To God be the glory for his mercy and Grace towards mankind… May his name be praise forever… Thanks to God and to all @ Paxherb. May God Almighty perfect the works of your hands
To God be the glory for this great achievement. Our prayer is that the healing power of the most high God will sanctify the drug to make it accomplish its purpose for the glory of His name. Amen.
I thank the Almighty GOD for HIS blessings on us Nevertheless let’s thread softly and with care We are told NAFDAC has approved Paxharbal Cugzin but there is no categorical statement that says that this said drug is a cure for COVID-19 Please enlighten us further
Thank God for this development. God will continue to strengthen you more. Pls i will like to be part of the distributors.
It’s interesting finding a relief drug on COVID as it has become a terrible challenges on human existence. I hope it will be available and affordable. Thanks to the researchers on their effort for this recorded achievement hoping to see more of this remarkable success in Nigeria.
Thank God for this great and successful move by the Rev father. At last, Nigeria has conquered covid -19 .
Glory be to God.
Pls, I will like to be one of your distributors in Lagos state, what are the procedures?my email is igbokonnenna@gmail.com
Good morning Fadakay please how can I get this Covid 19 drug and what is d market price I stay around Ajah area in Lagos state. Kindly email me d details of ur sales points in this vicinity thanks
Ojoba Gabriel
Hi I am so happy I found your weblog, I really found you by mistake, while I was looking on Askjeeve for something else, Regardless I
am here now and would just like to say kudos for a remarkable
post and a all round exciting blog (I also love the theme/design), I don’t
have time to look over it all at the moment but I
have bookmarked it and also added your RSS feeds,
so when I have time I will be back to read a lot more, Please
do keep up the fantastic job.